Clinical Evidence for Platelet-Rich Plasma PRP Injections
Findings Summary
- PRP treatment did not differ from placebo or sham treatment
- A Cochrane Collaboration review of PRP in all types of musculoskeletal injuries found very weak evidence for PRP decreasing bone pain in the short term, and no difference in patient functioning after fracture repair in the short-, medium- or long-term.
- As a result, the current consensus is that PRP injections may be useful in some cases of muscle damage, and is superior to cortisone injections over the long term, as evidenced in several studies.
- PRP compared to placebo at 3 months showed no difference, but slightly better pain scores in the PRP-treated groups at 6 months
- evidence was not sufficient to support the routine use of PRP as a conservative treatment in OA.

Risks and Considerations:
- Infection
- Injury to nerves or vessiles
- Cost varies and value is determined by payers
Platelet-rich plasma (PRP), also known as Autologous Conditioned Plasma (ACP) in the medical literature, is a non-cellular biologic product that has been touted for the treatment of a multitude of different conditions, with mixed clinical results. PRP is prepared from a patient’s whole blood sample by double centrifugation. Using commercially available kits and a standard centrifuge, the patient’s own (autologous) blood can be separated into three distinct fractions: (1) red blood cells, containing only erythrocytes, (2) platelet-poor plasma, containing mostly electrolytes and osmolytes, and (3) platelet-rich plasma which contain cytokines, large molecular weight enzymes, fibrin and cellular/acellular components like leukocytes and platelets. The platelet-rich (third) fraction can be further separated into the leukocyte-rich PRP (LR-PRP) fraction and the leukocyte-depleted PRP fractions (LD-PRP), the latter of which is also called leukocyte-reduced PRP (Lr-PRP) and “pure” PRP (pPRP). (In this report, we use the abbreviations “LR” and “LD” to denote the leukocyte-rich and leukocyte-depleted fractions, respectively). The distinction between the LR and LD fractions, which is often not given or recorded in medical literature, may be responsible, in part, for the mixed results of clinical trials. Additionally, the platelet concentration in either concentrate is often not reported, and varying clinical results may be due to subclinical “dosing” of platelets. In all cases, “true” PRP injection refers to the autologous injection of the third component with or without the other components.
Usually injected into the affected region of the same body (an auto-transfusion), PRP’s putative effects are mediated by the plethora of growth factors, cytokines, and excess platelets that can be found in the platelet-rich fraction. A summary of the platelet yield from commercially available PRP kits is given in Table 1. Reported cytokines that have been found within the PRP fraction include the following: platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), fibroblast growth factor (FGF), and insulin-like growth factors 1 and 2 (IGF-1 and IGF-2), among others. Because true PRP therapy involves the injection of the patient’s own plasma-derived product, there is no possibility of rejection or incompatible treatments. Clinical data on the effects of locally injected PRP is limited. PRP injections are used for a range of conditions, from musculoskeletal pain and injuries to cosmetic procedures.

Table 1. Platelet yields per device. *Clinaseal Sealed Technology Centrifuge; Salvin Dental Specialties Inc, Charlotte, NC. †ACE Surgical, Brockton, MA. ‡AG Curasan, Kleinostheim, Germany.
1. Musculoskeletal Uses
a. Tendon and Ligament Injuries.
PRP injections have been used to treat chronic tendon injuries such as elbow tendinitis (tennis elbow) and patellar tendinitis (jumper’s knee). The first clinical evaluation of PRP in these cases was published in the Journal of Clinical Medicine in 2002; that study showed that PRP lowered pain scores and increased elbow function when compared to physical therapy and therefore could be effective for elbow tendinopathy. The concentration of the platelets and healing factors such as epidermal growth factor (EGF) were correlated with the success of treatment, but whether or not a leukocyte-reduced or leukocyte-rich fraction was used was not delineated.
Subsequently, in 2018, a systematic review of medical literature and a meta-analysis of high-quality studies found that PRP was beneficial for the treatment of lateral epicondylitis. Moreover, numerous systematic reviews and meta-analyses have found that for elbow tendinopathy, PRP is superior to cortisone injections and has similar or equal effects compared to surgery. In 2021, a systematic review by the Cochrane Collaboration examining PRP and autologous whole-blood injections concluded that it was “uncertain” if PRP or autologous whole-blood injections improved elbow tendon healing. Meanwhile, in 2022, a meta-analysis of 26 studies evaluated the outcome of PRP treatments in elbow tendinopathy. According to that analysis, PRP-treated patients rated their results significantly better on validated patient-rated outcomes measures than patients treated with other means.
In 2009, a review article on PRP found few randomized controlled trials that adequately evaluated the safety and efficacy of PRP treatments for any condition, let alone Achilles tendinopathy, but did offer optimism that PRP could be useful. Xiao Chen and others analyzed subsequent studies between 2008 and 2019 in a review paper and came to the more assertive conclusion that PRP “may” be useful in such conditions (The American Journal of Sports Medicine. 46 (8): 2020–2032, July 2018). By 2019, however, the mood had shifted as a meta-analysis and review article from China found that, for most outcomes in Achilles tendinopathy, PRP treatment did not differ from placebo or sham treatment (Liu, C-J et al. Medicine. 98(16): e15278, April 2019). The systematic review undoubtedly mixed LD-PRP and LR-PRP results and encompassed the poorly controlled studies prior to 2009, as many studies did not specify which fraction (LD or LR) was used.
b. Post-surgical Bone Healing.
Clinicians first used PRP to accelerate healing after jaw or plastic surgeries, and indeed many notable reviews of PRP have been published in the dental literature (for instance, the Journal of Oral & Maxillofacial Surgery). An early paper by Richard E. Marx (J Oral Maxillofac Surg 62:489-496, 2004) reported that bone sites, for example, bone grafts, (as well as soft tissue sites) treated with PRP show favorable outcomes compared to non-treated bone graft sites in animal models. Regarding bone grafts, at 4 months following surgery, PRP showed favorable histology of trabecular bone density, bone maturity, and resorption remodeling.
These initial results held open the promise of rapid healing of bone grafts in sinus augmentation surgeries so that dental implants in patients with low-lying maxillary sinuses (an anatomic condition that otherwise increases the risk of infection and complications from dental implants) could be offered. However, a 2010 Cochrane Collaboration review of the use of PRP in sinus lifts during dental implant placement found no evidence of benefit, and, by 2014, a Cochrane Collaboration review of PRP in all types of musculoskeletal injuries found very weak evidence for PRP decreasing bone pain in the short term, and no difference in patient functioning after fracture repair in the short-, medium- or long-term. A 2016 review of PRP use to augment bone grafts found only one study reporting a difference in bone augmentation, while four studies found no difference. Therefore, PRP has not been shown to be a useful adjunct for bone healing in the facial bones, long bones, or vertebrae.
c. Muscular Injuries
PRP was applied to the problem of post-surgical healing following rotator cuff repair. Unlike the conclusions extracted from experience using PRP in peripheral load-bearing joints, the initial results from trials in the shoulder were generally negative. A 2009 review found few randomized controlled trials that adequately evaluated the safety and efficacy of PRP treatments and concluded that PRP was “a promising, but not proven, treatment option for joint, tendon, ligament, and muscle injuries”. While a 2018 review suggested that it may be useful, a 2019 review found it not to be useful in rotator cuff disease as PRP treatment did not differ from placebo treatment. This was the status quo for 2 years until new evidence emerged.
A prospective, non-randomized, unblinded study in 2021 examined the effectiveness of PRP for partial thickness rotator cuff tears. Patients were given 2 separate PRP injections and followed for 2 years. The study authors noted: “No adverse events were seen in any patient. Based on global rating scores, positive results were seen in 77.9% of patients at 6 months, 71.6% at 1 year, and 68.8% of patients at 2 years”. They found PRP most effective in more damaged tendons. In the same year (2021), a meta-analysis found that PRP was effective for partial rotator cuff tears, but the effects were no longer evident at 1 year. Just one year later (2022), a systematic review and meta-analysis again from China suggested improved patient-rated outcomes in patients with partial rotator cuff tears and effectiveness at 8 weeks post-injection. As a result, the current consensus is that PRP injections may be useful in some cases of muscle damage, and is superior to cortisone injections over the long term, as evidenced in several studies. This is currently an active area of research.
d. Osteoarthritis and Fasciitis
A meta-analysis by a group of physicians from Italy looked at data from 15 studies investigating PRP for plantar fasciitis that reported mean and standard deviations for pain and/or functional measure scales (M Franchini et al, Blood Transfusion [Trasfusione del Sangue]. 16(6): 502–513, November 2018). The most commonly reported measure was the AOFAS (American Orthopaedic Foot & Ankle Society) scale, but the authors displayed the VAS (Visual Analog Scale) of pain outcomes 3- and 6-months after PRP injection (Fig. 1). Very low-quality evidence (down-graded for serious risk of bias, inconsistency and imprecision) showed that PRP compared to placebo at 3 months showed no difference, but slightly better pain scores in the PRP-treated groups at 6 months. Likewise, in subgroup analyses of studies with steroids as a comparator, pooled data showed slightly better pain scores in PRP-treated groups at 6 months, but not at 3 months.
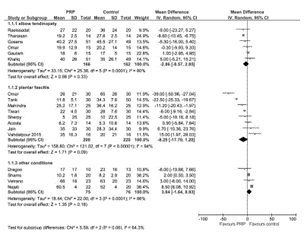
(a)
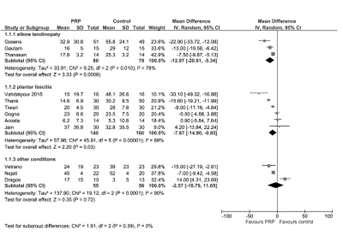
(b)
Figure 1. Forest Plots of pooled Visual Analogue Scale (VAS) data from PRP compared to controls at (a) 3 months and (b) 6 months from various trials in elbow tendinopathy and plantar fasciitis.
The use of PRP in osteoarthritis (OA) is an ongoing area of research. Tentative initial evidence supported the use of PRP in OA of the knee. A 2019 meta-analysis found that PRP “might” be more effective in reducing pain and improving function than hyaluronic acid in knee osteoarthritis, but like the comparison to other conservative treatments for non-surgical orthopedic illnesses (e.g. steroid injection for plantar fasciitis), evidence was not sufficient to support the routine use of PRP as a conservative treatment in OA.
2. Uses in Dermatology and Cosmetic Procedures
A. Androgenetic Alopecia / Hair Loss
PRP injections are reportedly possibly (“can be”) effective in treating male pattern baldness, both in preventing hair loss and promoting new hair growth. PRP can also aid in the stimulation of hair growth after hair transplants in animal models. Yet, in a 2013 review paper (I de Sousa and A Tosti, Expert Opinion on Investigational Drugs, 22 (5): 573–89, May 2013) the authors suggested more evidence was needed to determine the effectiveness of hair regrowth. Despite this lack of evidence, commercial clinics have started treating androgenetic alopecia with PRP grafts and added an optional “PRP step” to hair treatments, nominally as a means to address this lack of evidence. V Pathania et al have even gone so far as to outline a single-handed (presumably more efficient) technique for performing PRP during treatments associated with hair restoration such as follicular unit extraction (FUE) and hair transplantation (Journal of the American Academy of Dermatology. 84(2):e77-8, Feb 2021).
B. Skin Rejuvenation
In the previously mentioned early paper by Richard E. Marx (J Oral Maxillofac Surg 62:489-496, 2004), Dr Marx reported the effect of PRP treatment on soft tissue sites (for example, split-thickness skin graft donor sites) in addition to the bone grafts. Histological and clinical images show favorable outcomes of PRP-treated soft tissue sites, compared to thrombin-treated sites within the same individual. Regarding skin graft sites, thrombin-treated areas show less evidence of epithelialization and more peripheral erythema at 6 days, and ultimately resulted in “scarring, [skin] contraction, and… a greater variation in pigmentation” at 6 months. Thus, there was hope for the use of PRP as an “adjunct” to plastic surgery for skin rejuvenation.
As this industry is largely unregulated, and practiced by multiple specialties (dermatology, plastic surgery, aestheticians, and so forth), this initial data gave rise to a cottage industry of “vampire facials,” wherein “customers” (patients) would pay an average cost of about $1,300 for skin rejuvenation. The main expense involved in this procedure is “micro-needling,” as the area-to-amount over which PRP is to be injected is very large and precludes the use of small-gauge needles. Microneedling accounts for around $800 of the total cost. The next most important cost center is the PRP therapy fee, at around $500, which involves the extraction and application of centrifuged and fractionated blood as given in the Introduction. Commercially-minded clinics now advertise PRP treatments for the lucrative adjunct treatment of various conditions, mainly targeting the older population. Such clinics often state that they do not need FDA approval to perform these procedures because the cells are harvested and returned to the patient during “the same surgical procedure,” thus negating the need for a biological license. Although the FDA states that it disagrees with that position, in 2020, only a handful of clinics received warnings about deceptive advertising practices or the false representation of medical claims.
Referencing one of those aforementioned clinics (https://hbmag.com/introducing-vampire/), the purported list of procedures or conditions meant to be aided by PRP injection includes:
- facelifts (using hyaluronic acid or adipose tissue) and filler-free facials,
- breast lifts,
- female sexual dysfunction (“O-shot”), loss of libido (“P-shot”), and male erectile dysfunction (resulting from the after-effects of surgery, drugs, or diabetes)
- acne scars,
- axillary hyperhidrosis,
- hyperpigmentation conditions like melasma, periorbital melanosis, and striae distensae,
While the medical literature abounds with case reports of “horror stories” of such procedures gone awry, there is also a fair amount of evidence detailing the use of PRP in such cosmetic and dermatologic procedures. More recently, authors have begun to ask, rightly, “Where Is the Evidence [for the efficacy of PRP treatment in these conditions]?” (NV Goddard and N Waterhouse, Aesthetic Surgery Journal, 40(4): 460–465, April 2020) as no topical systematic review or meta-analysis of the data has been published.

